Publications
You can search by author, year, title and/or journal. If you don´t find what you need, please contact us.
Total publications
80. Click in every publication for more information.
Pages:
 1 2 3 4
1 2 3 4 
2013

Ruiz-Dueñas FJ, Lundell T, Floudas D, Nagy LG, Barrasa JM, Hibbett DS, Martínez AT (2013).
"Lignin-degrading peroxidases in Polyporales: an evolutionary survey based on 10 sequenced genomes". Mycologia, 105: 1428-1444.The genomes of three representative Polyporales (Bjerkandera adusta, Phlebia brevispora and a member of the Ganoderma lucidum complex) were sequenced to expand our knowledge on the diversity of ligninolytic and related peroxidase genes in this Basidiomycota order that includes most wood-rotting fungi. The survey was completed by analyzing the heme-peroxidase genes in the already available genomes of seven more Polyporales species representing the antrodia, gelatoporia, core polyporoid and phlebioid clades. The study confirms the absence of ligninolytic peroxidase genes from the manganese peroxidase (MnP), lignin peroxidase (LiP) and versatile peroxidase (VP) families, in the brown-rot fungal genomes (all of them from the antrodia clade), which include only a limited number of predicted low redox-potential generic peroxidase (GP) genes. When members of the heme-thiolate peroxidase (HTP) and dye-decolorizing peroxidase (DyP) superfamilies (up to a total of 64 genes) also are considered, the newly sequenced B. adusta appears as the Polyporales species with the highest number of peroxidase genes due to the high expansion of both the ligninolytic peroxidase and DyP (super)families. The evolutionary relationships of the 111 genes for class-II peroxidases (from the GP, MnP, VP, LiP families) in the 10 Polyporales genomes is discussed including the existence of different MnP subfamilies and of a large and homogeneous LiP cluster, while different VPs mainly cluster with short MnPs. Finally, ancestral state reconstructions showed that a putative MnP gene, derived from a primitive GP that incorporated the Mn(II)-oxidation site, is the precursor of all the class-II ligninolytic peroxidases. Incorporation of an exposed tryptophan residue involved in oxidative degradation of lignin in a short MnP apparently resulted in evolution of the first VP. One of these ancient VPs might have lost the Mn(II)-oxidation site being at the origin of all the LiP enzymes, which are found only in species of the order Polyporales.
External web link
Salvachúa D, Martínez AT, Tien M, López-Lucendo MF, García F, de los Ríos V, Martínez MJ, Prieto A (2013).
"Differential proteomic analysis of the secretome of Irpex lacteus and other white-rot fungi during wheat straw pretreatment". Biotechnology for Biofuels, 6: 115-129.Background
Identifying new high-performance enzymes or enzyme complexes to enhance biomass degradation is the key for the development of cost-effective processes for ethanol production. Irpex lacteus is an efficient microorganism for wheat straw pretreatment, yielding easily hydrolysable products with high sugar content. Thus, this fungus was selected to investigate the enzymatic system involved in lignocellulose decay, and its secretome was compared to those from Phanerochaete chrysosporium and Pleurotus ostreatus which produced different degradation patterns when growing on wheat straw. Extracellular enzymes were analyzed through 2D-PAGE, nanoLC/MS-MS, and homology searches against public databases.
Results
In wheat straw, I. lacteus secreted proteases, dye-decolorizing and manganese-oxidizing peroxidases, and H2O2 producing-enzymes but also a battery of cellulases and xylanases, excluding those implicated in cellulose and hemicellulose degradation to their monosaccharides, making these sugars poorly available for fungal consumption. In contrast, a significant increase of β-glucosidase production was observed when I. lacteus grew in liquid cultures. P. chrysosporium secreted more enzymes implicated in the total hydrolysis of the polysaccharides and P. ostreatus produced, in proportion, more oxidoreductases.
Conclusion
The protein pattern secreted during I. lacteus growth in wheat straw plus the differences observed among the different secretomes, justify the fitness of I. lacteus for biopretreatment processes in 2G-ethanol production. Furthermore, all these data give insight into the biological degradation of lignocellulose and suggest new enzyme mixtures interesting for its efficient hydrolysis.
Keywords:
Enzymatic hydrolysis; Bioethanol; DyP;
Pleurotus ostreatus;
Phanerochaete chrysosporium; Lignocellulose; Extracellular enzymes
External web link
Salvachúa D, Prieto A, Martínez AT, Martínez MJ (2013).
"Characterization of a novel dye-decolorizing peroxidase (DyP)-type enzyme from Irpex lacteus and its application in enzymatic hydrolysis of wheat straw". Appl. Environ. Microbiol., 79: 4316-4324.Irpex lacteus is a white rot basidiomycete proposed for a wide spectrum of biotechnological applications which presents an interesting, but still scarcely known, enzymatic oxidative system. Among these enzymes, the production, purification, and identification of a new dye-decolorizing peroxidase (DyP)-type enzyme, as well as its physico-chemical, spectroscopic, and catalytic properties, are described in the current work. According to its N-terminal sequence and peptide mass fingerprinting analyses,
I. lacteus DyP showed high homology (>95%) with the hypothetical (not isolated or characterized) protein cpop21 from an unidentified species of the family Polyporaceae. The enzyme had a low optimal pH, was very stable to acid pH and temperature, and showed improved activity and stability at high H
2O
2 concentrations compared to other peroxidases. Other attractive features of
I. lacteus DyP were its high catalytic efficiency oxidizing the recalcitrant anthraquinone and azo dyes assayed (kcat/Km of 1.6 × 10(6) s(-1) M(-1)) and its ability to oxidize nonphenolic aromatic compounds like veratryl alcohol. In addition, the effect of this DyP during the enzymatic hydrolysis of wheat straw was checked. The results suggest that
I. lacteus DyP displayed a synergistic action with cellulases during the hydrolysis of wheat straw, increasing significantly the fermentable glucose recoveries from this substrate. These data show a promising biotechnological potential for this enzyme.
External web link
Salvachúa D, Prieto A, Mattinen ML, Tamminen T, Liitiä T, Lille M, Willför S, Martínez AT, Martínez MJ, Faulds CB (2013).
"Versatile peroxidase as a valuable tool for generating new biomolecules by homogeneous and heterogeneous cross-linking". Enz. Microb. Technol., 52: 303-311.The modification and generation of new biomolecules intended to give higher molecular-mass species for biotechnological purposes, can be achieved by enzymatic cross-linking. The versatile peroxidase (VP) from Pleurotus eryngii is a high redox-potential enzyme with oxidative activity on a wide variety of substrates. In this study, VP was successfully used to catalyze the polymerization of low molecular mass compounds, such as lignans and peptides, as well as larger macromolecules, such as protein and complex polysaccharides. Different analytical, spectroscopic, and rheological techniques were used to determine structural changes and/or variations of the physicochemical properties of the reaction products. The lignans secoisolariciresinol and hydroxymatairesinol were condensed by VP forming up to 8 unit polymers in the presence of organic co-solvents and Mn(2+). Moreover, 11 unit of the peptides YIGSR and VYV were homogeneously cross-linked. The heterogeneous cross-linking of one unit of the peptide YIGSR and several lignan units was also achieved. VP could also induce gelation of feruloylated arabinoxylan and the polymerization of β-casein. These results demonstrate the efficacy of VP to catalyze homo- and hetero-condensation reactions, and reveal its potential exploitation for polymerizing different types of compounds.
External web link
Strittmatter E, Liers C, Ullrich R, Wachter S, Hofrichter M, Plattner D, Piontek K (2013).
"First Crystal Structure of a Fungal High-Redox Potential Dye-decolorizing Peroxidase: Substrate Interaction Sites and Long-Range Electron Transfer". J. Biol. Chem., 288: 4095-4102.DyP-type peroxidases (DyP = dye decolorizing peroxidases) belong to the large group of heme peroxidases. They utilize hydrogen peroxide to catalyze oxidations of various organic compounds. AauDyPI from Auricularia auricula-judae (Fungi) was crystallized and its crystal structure was determined at 2.1 A resolution. The mostly helical structure also shows a beta-sheet motif typical for DyPs and Cld-related structures and includes the complete poypeptide chain. At the distal side of the heme molecule, a flexible aspartate residue (Asp168) plays a key role in catalysis. It guides incoming hydrogen peroxide toward the heme iron and mediates proton rearrangement in the process of Compound I formation. Afterwards, its side chain changes its conformation now pointing toward the protein backbone. We propose an extended functionality of Asp168, that acts like a gatekeeper by altering the width of the heme cavity access channel. Chemical modifications of potentially redox-active amino acids show that a tyrosine is involved in substrate interaction. Using spin trapping experiments a transient radical on the surface-exposed Tyr337 was identified as the oxidation site for bulky substrates. A possible long-range electron transfer (LRET) pathway from the surface of the enzyme to the redox cofactor (heme) is discussed.
External web link
Strittmatter E, Wachter S, Liers C, Ullrich R, Hofrichter M, Plattner D, Piontek K (2013).
"Radical formation on a conserved tyrosine residue is crucial for DyP activity". Arch. Biochem. Biophys., 537: 161-167.Dye-decolorizing peroxidases (DyPs) are able to cleave bulky anthraquinone dyes. The recently published crystal structure of AauDyPI reveals that a direct oxidation in the distal heme cavity can be excluded for most DyP substrates. It is shown that a surface-exposed tyrosine residue acts as a substrate interaction site for bulky substrates. This amino acid is conserved in eucaryotic DyPs but is missing in the structurally related chlorite dismutases (Clds). Dye-decolorizing peroxidases of procaryotic origin equally possess a conserved tyrosine in the same region of the polypeptide albeit not at the homologous position.
External web link
Wang X, Peter S, Ullrich R, Hofrichter M, Groves JT (2013).
"Driving Force for Oxygen-Atom Transfer by Heme-Thiolate Enzymes". Angew. Chem. Int. Ed., 52: 9238-9241.Redox potentials for three redox couples in AaeAPO-catalyzed reactions have been measured, thus placing these heme-thiolate reactive intermediates on an absolute energy scale for the first time. The importance of the axial thiolate ligand and the basic nature of compound II ferryl oxygen atom are discussed in terms of these redox potentials.
External web link
2012

Camarero S, Pardo I, Cañas AI, Molina-Espeja P, Record E, Martínez AT, Pecyna MJ, Alcalde M (2012).
"Engineering platforms for directed evolution of Laccase from Pycnoporus cinnabarinus". Appl. Environ. Microbiol., 78: 1370-1384.While the Pycnoporus cinnabarinus laccase (PcL) is one of the most promising high-redox-potential enzymes for environmental biocatalysis, its practical use has to date remained limited due to the lack of directed evolution platforms with which to improve its features. Here, we describe the construction of a PcL fusion gene and the optimization of conditions to induce its functional expression in Saccharomyces cerevisiae, facilitating its directed evolution and semirational engineering. The native PcL signal peptide was replaced by the α-factor preproleader, and this construct was subjected to six rounds of evolution coupled to a multiscreening assay based on the oxidation of natural and synthetic redox mediators at more neutral pHs. The laccase total activity was enhanced 8,000-fold: the evolved α-factor preproleader improved secretion levels 40-fold, and several mutations in mature laccase provided a 13.7-fold increase in k(cat). While the pH activity profile was shifted to more neutral values, the thermostability and the broad substrate specificity of PcL were retained. Evolved variants were highly secreted by Aspergillus niger (∼23 mg/liter), which addresses the potential use of this combined-expression system for protein engineering. The mapping of mutations onto the PcL crystal structure shed new light on the oxidation of phenolic and nonphenolic substrates. Furthermore, some mutations arising in the evolved preproleader highlighted its potential for heterologous expression of fungal laccases in yeast (S. cerevisiae).
External web link
Fernandez-Fueyo E, Ruiz-Dueñas FJ, Ferreira P, .... , Martínez AT, Vicuña R, Cullen D (2012).
"Comparative genomics of Ceriporiopsis subvermispora and Phanerochaete chrysosporium provide insight into selective ligninolysis". Proc. Natl. Acad. Sci. USA, 109: 5458-5463.Efficient lignin depolymerization is unique to the wood decay basidiomycetes, collectively referred to as white rot fungi.
Phanerochaete chrysosporium simultaneously degrades lignin and cellulose, whereas the closely related species,
Ceriporiopsis subvermispora, also depolymerizes lignin but may do so with relatively little cellulose degradation. To investigate the basis for selective ligninolysis, we conducted comparative genome analysis of
C. subvermispora and
P. chrysosporium. Genes encoding manganese peroxidase numbered 13 and five in
C. subvermispora and
P. chrysosporium, respectively. In addition, the
C. subvermispora genome contains at least seven genes predicted to encode laccases, whereas the
P. chrysosporium genome contains none. We also observed expansion of the number of
C. subvermispora desaturase-encoding genes putatively involved in lipid metabolism. Microarray-based transcriptome analysis showed substantial up-regulation of several desaturase and MnP genes in wood-containing medium. MS identified MnP proteins in
C. subvermispora culture filtrates, but none in
P. chrysosporium cultures. These results support the importance of MnP and a lignin degradation mechanism whereby cleavage of the dominant nonphenolic structures is mediated by lipid peroxidation products. Two
C. subvermispora genes were predicted to encode peroxidases structurally similar to
P. chrysosporium lignin peroxidase and, following heterologous expression in
Escherichia coli, the enzymes were shown to oxidize high redox potential substrates, but not Mn
2+. Apart from oxidative lignin degradation, we also examined cellulolytic and hemicellulolytic systems in both fungi. In summary, the
C. subvermispora genetic inventory and expression patterns exhibit increased oxidoreductase potential and diminished cellulolytic capability relative to
P. chrysosporium.
External web link
Fernandez-Fueyo E, Ruiz-Dueñas FJ, Miki Y, Martínez MJ, Hammel KE, Martínez AT (2012).
"Lignin-degrading peroxidases from the genome of the selective ligninolytic fungus Ceriporiopsis subvermispora". J. Biol. Chem., 287: 16903-16916.The white-rot fungus Ceriporiopsis subvermispora delignifies lignocellulose with high selectivity, but until now has appeared to lack the specialized peroxidases, termed lignin peroxidases (LiPs) and versatile peroxidases (VPs), that are generally thought important for ligninolysis. We screened the recently sequenced C. subvermispora genome for genes that encode peroxidases with a potential ligninolytic role. A total of 26 peroxidase genes was apparent after a structural-functional classification based on homology modeling and a search for diagnostic catalytic amino acid residues. In addition to revealing the presence of nine heme-thiolate peroxidase superfamily members and the unexpected absence of the dye-decolorizing peroxidase superfamily, the search showed that the C. subvermispora genome encodes 16 Class II enzymes in the plant-fungal-bacterial peroxidase superfamily, where LiPs and VPs are classified. The 16 encoded enzymes include 13 putative manganese peroxidases and one generic peroxidase, but most notably two peroxidases containing the catalytic tryptophan characteristic of LiPs and VPs. We expressed these two enzymes in Escherichia coli and determined their substrate specificities on typical LiP/VP substrates, including nonphenolic lignin model monomers and dimers, as well as synthetic lignin. The results show that the two newly discovered C. subvermispora peroxidases are functionally competent LiPs, while also suggesting that they are phylogenetically and catalytically intermediate between classical LiPs and VPs. These results offer new insight into selective lignin degradation by C. subvermispora.
External web link
Floudas D, .... , Martínez AT, .... , Ferreira P, .... , Ruiz-Dueñas FJ, .... , Hibbett DS (2012).
"The Paleozoic Origin of Enzymatic Lignin Decomposition Reconstructed from 31 Fungal Genomes". Science, 336: 1715-1719.Wood is a major pool of organic carbon that is highly resistant to decay, owing largely to the presence of lignin. The only organisms capable of substantial lignin decay are white rot fungi in the Agaricomycetes, which also contains non–lignin-degrading brown rot and ectomycorrhizal species. Comparative analyses of 31 fungal genomes (12 generated for this study) suggest that lignin-degrading peroxidases expanded in the lineage leading to the ancestor of the Agaricomycetes, which is reconstructed as a white rot species, and then contracted in parallel lineages leading to brown rot and mycorrhizal species. Molecular clock analyses suggest that the origin of lignin degradation might have coincided with the sharp decrease in the rate of organic carbon burial around the end of the Carboniferous period.
External web link
García-Ruiz E, González-Pérez D, Ruiz-Dueñas FJ, Martínez AT, Alcalde M (2012).
"Directed evolution of a temperature, peroxide and alkaline pH tolerant versatile peroxidase". Biochem. J., 441: 487-498.The versatile peroxidases (VPs) secreted by white-rot fungi are fundamental for the natural decay of lignin. In this study, a fusion gene containing the VP from Pleurotus eryngii was subjected to six rounds of directed evolution, achieving a level of secretion in Saccharomyces cerevisiae (21 mg/L) as yet unseen for any ligninolytic peroxidase. The evolved variant for expression harboured 4 mutations and increased its total VP activity 129-fold. The signal leader processing by the STE13 protease at the Golgi compartment changed as consequence of over-expression, retaining the additional N-terminal sequence EAEA that enhanced secretion. The engineered N-terminal truncated variant displayed similar biochemical properties as the non-truncated counterpart in terms of kinetics, stability and spectroscopic features. Additional cycles of evolution raised the T50 8ºC and significantly increased the enzyme stability at alkaline pHs. In addition, the Km for H2O2 was enhanced up to 15-fold while the catalytic efficiency was maintained, and there was an improvement in peroxide stability (with half lives for H2O2 of 43 min at a H2O2/enzyme molar ratio of 4000:1). Overall, the directed evolution approach described provides a set of strategies for selecting VPs with improvements in secretion, activity and stability.
External web link
González-Pérez D, García-Ruiz E, Alcalde M (2012).
"Saccharomyces cerevisiae in directed evolution: An efficient tool to improve enzymes". Bioengineered, 3: 1-6.Over the past 20 years, directed evolution has been seen to be the most reliable approach to protein engineering. Emulating the natural selection algorithm, ad hoc enzymes with novel features can be tailor-made for practical purposes through iterative rounds of random mutagenesis, DNA recombination and screening. Of the heterologous hosts used in laboratory evolution experiments, the budding yeast
Saccharomyces cerevisiae has become the best choice to express eukaryotic proteins with improved properties.
S. cerevisiae not only allows mutant enzymes to be secreted but also, it permits a wide range of genetic manipulations to be employed, ranging from in vivo cloning to the creation of greater molecular diversity, thanks to its efficient DNA recombination apparatus. Here, we summarize some successful examples of the use of the
S. cerevisiae machinery to accelerate artificial evolution, complementing the traditional in vitro methods to generate tailor-made enzymes.
External web link
Hernández-Ortega A, Ferreira P, Martínez AT (2012).
"Fungal aryl-alcohol oxidase: a peroxide-producing flavoenzyme involved in lignin degradation". Appl. Microbiol. Biotechnol., 93: 1395-1410.Aryl-alcohol oxidase (AAO) is an extracellular flavoprotein providing the H(2)O(2) required by ligninolytic peroxidases for fungal degradation of lignin, the key step for carbon recycling in land ecosystems. O(2) activation by Pleurotus eryngii AAO takes place during the redox-cycling of p-methoxylated benzylic metabolites secreted by the fungus. Only Pleurotus AAO sequences were available for years, but the number strongly increased recently due to sequencing of different basidiomycete genomes, and a comparison of 112 GMC (glucose-methanol-choline oxidase) superfamily sequences including 40 AAOs is presented. As shown by kinetic isotope effects, alcohol oxidation by AAO is produced by hydride transfer to the flavin, and hydroxyl proton transfer to a base. Moreover, site-directed mutagenesis studies showed that His502 activates the alcohol substrate by proton abstraction, and this result was extended to other GMC oxidoreductases where the nature of the base was under discussion. However, in contrast with that proposed for GMC oxidoreductases, the two transfers are not stepwise but concerted. Alcohol docking at the buried AAO active site resulted in only one catalytically relevant position for concerted transfer, with the pro-R α-hydrogen at distance for hydride abstraction. The expected hydride-transfer stereoselectivity was demonstrated, for the first time in a GMC oxidoreductase, by using the (R) and (S) enantiomers of α-deuterated p-methoxybenzyl alcohol. Other largely unexplained aspects of AAO catalysis (such as the unexpected specificity on substituted aldehydes) can also be explained in the light of the recent results. Finally, the biotechnological interest of AAO in flavor production is extended by its potential in production of chiral compounds taking advantage from the above-described stereoselectivity.
External web link
Hernández-Ortega A, Ferreira P, Merino P, Medina M, Guallar V, Martínez AT (2012).
"Stereoselective Hydride Transfer by Aryl-Alcohol Oxidase, a Member of the GMC Superfamily". ChemBioChem, 13: 427-435.Primary alcohol oxidation by aryl-alcohol oxidase (AAO), a flavoenzyme providing H2O2 to ligninolytic peroxidases, is produced by concerted proton and hydride transfers, as shown by substrate and solvent kinetic isotope effects (KIEs). Interestingly, when the reaction was investigated with synthesized (R)- and (S)-α-deuterated p-methoxybenzyl alcohol, a primary KIE (≈6) was observed only for the Renantiomer, revealing that the hydride transfer is highly stereoselective. Docking of p-methoxybenzyl alcohol at the buried crystal active site, together with QM/MM calculations, showed that this stereoselectivity is due to the position of the hydride- and proton-receiving atoms (flavin N5 and His502 Nε, respectively) relative to the alcohol Cα-substituents, and to the concerted nature of transfer (the pro-S orientation corresponding to a 6 kcal mol−1 penalty with respect to the pro-R orientation). The role of His502 is supported by the lower activity (by three orders of magnitude) of the H502A variant. The above stereoselectivity was also observed, although activities were much lower, in AAO reactions with secondary aryl alcohols (over 98 % excess of the R enantiomer after treatment of racemic 1-(p-methoxyphenyl)ethanol, as shown by chiral HPLC) and especially with use of the F501A variant. This variant has an enlarged active site that allow better accommodation of the α-substituents, resulting in higher stereoselectivity (S/R ratios) than is seen with AAO. High enantioselectivity in a member of the GMC oxidoreductase superfamily is reported for the first time, and shows the potential for engineering of AAO for deracemization purposes.
External web link
Hernández-Ortega A, Lucas F, Ferreira P, Medina M, Guallar V, Martínez AT (2012).
"Role of Active Site Histidines in the Two Half-Reactions of the Aryl-Alcohol Oxidase Catalytic Cycle". Biochemistry, 51: 6595-6608.The crystal structure of aryl-alcohol oxidase (AAO), a flavoenzyme involved in lignin degradation, reveals two active-site histidines, whose role in the two enzyme half-reactions was investigated. The redox state of flavin during turnover of the variants obtained show a stronger histidine involvement in the reductive than in the oxidative half-reaction. This was confirmed by the
kcat/
Km(Al) and reduction constants that are 2-3 orders of magnitude decreased for the His546 variants and up to 5 orders for the His502 variants, while the corresponding O
2 constants only decreased up to 1 order of magnitude. These results confirm His502 as the catalytic base in the AAO reductive half-reaction. The solvent kinetic isotope effect (KIE) revealed that hydroxyl proton abstraction is partially limiting the reaction, while the α-deuterated alcohol KIE showed a stereoselective hydride transfer. Concerning the oxidative half-reaction, directed mutagenesis and computational simulations indicate that only His502 is involved. Quantum mechanical/molecular mechanical (QM/MM) reveals an initial partial electron transfer from the reduced FADH
– to O
2, without formation of a flavin-hydroperoxide intermediate. Reaction follows with a nearly barrierless His502H
+ proton transfer that decreases the triplet/singlet gap. Spin inversion and second electron transfer, concomitant with a slower proton transfer from flavin N5, yields H
2O
2. No solvent KIE was found for O
2 reduction confirming that the His502 proton transfer does not limit the oxidative half-reaction. However, the small KIE on
kcat/
Km(Ox), during steady-state oxidation of α-deuterated alcohol, suggests that the second proton transfer from N5H is partially limiting, as predicted by the QM/MM simulations.
External web link
Kluge M, Ullrich R, Scheibner K, Hofrichter M (2012).
"Stereoselective benzylic hydroxylation of alkylbenzenes and epoxidation of styrene derivatives catalyzed by the peroxygenase of Agrocybe aegerita". Green Chem., 14: 440-446.Here we report on the stereoselective benzylic hydroxylation and C1–C2 epoxidation of alkylbenzenes and styrene derivatives, respectively, by a heme-thiolate peroxygenase (EC 1.11.2.1) from the fungus
Agrocybe aegerita. Benzylic hydroxylation led exclusively to the (
R)-1-phenylalkanols. For (
R)-1-phenylethanol, (
R)-1-phenylpropanol and (
R)-1-tetralol, the ee reached >99%. For longer chain lengths, the enantiomeric excesses (ee) and total turnover numbers (TTN) decreased while the number of by-products,
e.g. 1-phenylketones, increased. Epoxidation of straight chain and cyclic styrene derivatives gave a heterogeneous picture and resulted in moderate to excellent ee values and TTN:
e.g., in the case of (1
R,2
S)-
cis-β-methylstyrene oxide formation, an ee >99% and a TTN of 110

000 was achieved. Hydroxylation and epoxidation were true peroxygenations, which was demonstrated by the incorporation of
18O from H
218O
2 into the products. The use of fed-batch devices and varying feeding strategies for the substrate and co-substrate turned out to be a suitable approach to optimize peroxygenase catalysis.
External web link
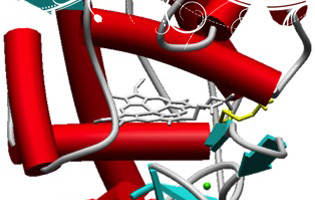
Morales M, Mate MJ, Romero A, Martínez MJ, Martínez AT, Ruiz-Dueñas FJ (2012).
"Two oxidation sites for low redox-potential substrates: A directed mutagenesis, kinetic and crystallographic study on Pleurotus eryngii versatile peroxidase". J. Biol. Chem., 287: 41053-41067.Versatile peroxidase shares with manganese peroxidase and lignin peroxidase the ability to oxidize Mn2+ and high redox-potential aromatic compounds, respectively. Moreover, it is also able to oxidize phenols (and low redox-potential dyes) at two catalytic sites, as shown by biphasic kinetics. A high-efficiency site (with 2,6-dimethoxyphenol and p-hydroquinone catalytic efficiencies of ~70 and ~700 s-1 mM-1, respectively) was localized at the same exposed Trp164 responsible for high redox-potential substrate oxidation (as shown by activity loss in the W164S variant). The second site, characterized by low catalytic efficiency (~3 and ~50 s-1 mM-1 for 2,6-dimethoxyphenol and p-hydroquinone, respectively) was localized at the main heme access-channel. Steady-state and transient-state kinetics for oxidation of phenols and dyes at the latter site were improved when side-chains of residues forming the heme channel edge were removed in single and multiple variants. Among them, the E140G/K176G, E140G/P141G/K176G and E140G/W164S/K176G variants attained catalytic efficiencies for oxidation of 2,2´-azino-bis(3-ethylbenzothiazoline-6-sulfonate) at the heme channel similar to those of the exposed tryptophan site. The heme channel enlargement shown by X-ray diffraction of the E140G, P141G, K176G and E140G/K176G variants would allow a better substrate accommodation near the heme, as revealed by the up to 26-fold lower Km values (compared with native VP). The resulting interactions were shown by the X-ray structure of the E140G-guaiacol complex, which includes two H-bonds of the substrate with Arg43 and Pro139 in the distal heme pocket (at the end of the heme channel) and several hydrophobic interactions with other residues and the heme cofactor.
External web link
Poraj-Kobielska M, Kinne M, Ullrich R, Scheibner K, Hofrichter M (2012).
"A spectrophotometric assay for the detection of fungal peroxygenases". Anal. Biochem., 421: 327-329.Rapid and simple spectrophotometric methods are required for the unambiguous detection of recently discovered fungal peroxygenases
in vivo and
in vitro. This paper describes a peroxygenase-specific assay using 5-nitro-1,3-benzodioxole as substrate. The product, 4-nitrocatechol, produces a yellow color at pH 7, which can be followed over time at 425 nm (
ε425 = 9,700 M
−1 cm
−1), and a red color when adjusted to pH >12, which can be measured in form of an end-point determination at 514 nm (
ε514 = 11,400 M
−1 cm
−1). The assay is suitable for detecting peroxygenase activities in complex growth media and environmental samples as well as for high-throughput screenings.
External web link
Wang X, Peter S, Kinne M, Hofrichter M, Groves JT (2012).
"Detection and Kinetic Characterization of a Highly Reactive Heme–Thiolate Peroxygenase Compound I". J. Am. Chem. Soc., 134: 12897-12900.The extracellular heme-thiolate peroxygenase from
Agrocybe aegerita (
AaeAPO) has been shown to hydroxylate alkanes and numerous other substrates using hydrogen peroxide as the terminal oxidant. We describe the kinetics of formation and decomposition of
AaeAPO compound I upon its reaction with
mCPBA. The UV–vis spectral features of
AaeAPO-I (361, 694 nm) are similar to those of chloroperoxidase-I and the recently described cytochrome P450-I. The second-order rate constant for
AaeAPO-I formation was 1.0 (±0.4) × 10
7 M
–1 s
–1 at pH 5.0, 4 °C. The relatively slow decomposition rate, 1.4 (±0.03) s
–1, allowed the measurement of its reactivity toward a panel of substrates. The observed rate constants,
k2′, spanned 5 orders of magnitude and correlated linearly with bond dissociation enthalpies (BDEs) of strong C–H bond substrates with a log
k2′ vs BDE slope of

0.4. However, the hydroxylation rate was insensitive to a C–H BDE below 90 kcal/mol, similar to the behavior of the
tert-butoxyl radical. The shape and slope of the Brønsted–Evans–Polanyi plot indicate a symmetrical transition state for the stronger C–H bonds and suggest entropy control of the rate in an early transition state for weaker C–H bonds. The
AaeAPO-II Fe
IVO–H BDE was estimated to be

103 kcal/mol. All results support the formation of a highly reactive
AaeAPO oxoiron(IV) porphyrin radical cation intermediate that is the active oxygen species in these hydroxylation reactions.
External web link

 1 2 3 4
1 2 3 4 


 000 was achieved. Hydroxylation and epoxidation were true peroxygenations, which was demonstrated by the incorporation of 18O from H218O2 into the products. The use of fed-batch devices and varying feeding strategies for the substrate and co-substrate turned out to be a suitable approach to optimize peroxygenase catalysis.
000 was achieved. Hydroxylation and epoxidation were true peroxygenations, which was demonstrated by the incorporation of 18O from H218O2 into the products. The use of fed-batch devices and varying feeding strategies for the substrate and co-substrate turned out to be a suitable approach to optimize peroxygenase catalysis.
 0.4. However, the hydroxylation rate was insensitive to a C–H BDE below 90 kcal/mol, similar to the behavior of the tert-butoxyl radical. The shape and slope of the Brønsted–Evans–Polanyi plot indicate a symmetrical transition state for the stronger C–H bonds and suggest entropy control of the rate in an early transition state for weaker C–H bonds. The AaeAPO-II FeIVO–H BDE was estimated to be
0.4. However, the hydroxylation rate was insensitive to a C–H BDE below 90 kcal/mol, similar to the behavior of the tert-butoxyl radical. The shape and slope of the Brønsted–Evans–Polanyi plot indicate a symmetrical transition state for the stronger C–H bonds and suggest entropy control of the rate in an early transition state for weaker C–H bonds. The AaeAPO-II FeIVO–H BDE was estimated to be